FamiCord Group
Cledar developed a CRM base, along with a lab monitoring and failure detection system for FamiCord, enabling our client to get the AABB accreditation.
-
Country
Poland, Hungary
-
Industry
Healthcare
-
Duration
2009 - 2011
Overview
FamiCord is Europe’s leading cord blood bank, having stored 500,000+ medical sample units for customers from all over Europe. The process of acquiring biomaterial starts with collecting the blood of the cord and placenta during childbirth, together with a few test sample vials. FamiCord records personal and health-related details of the mother and child, incl. test results. Our client monitors stored material in the patient’s database, manages billing, and upon patient’s request, provides access to the stored blood for medical procedures, like treatments for leukemia and other forms of cancer.
Overview
40,000+ medical records
and medical samples in the database
13 laboratories
located in different parts of Europe
1500+ partner hospitals
around Europe
Challenge
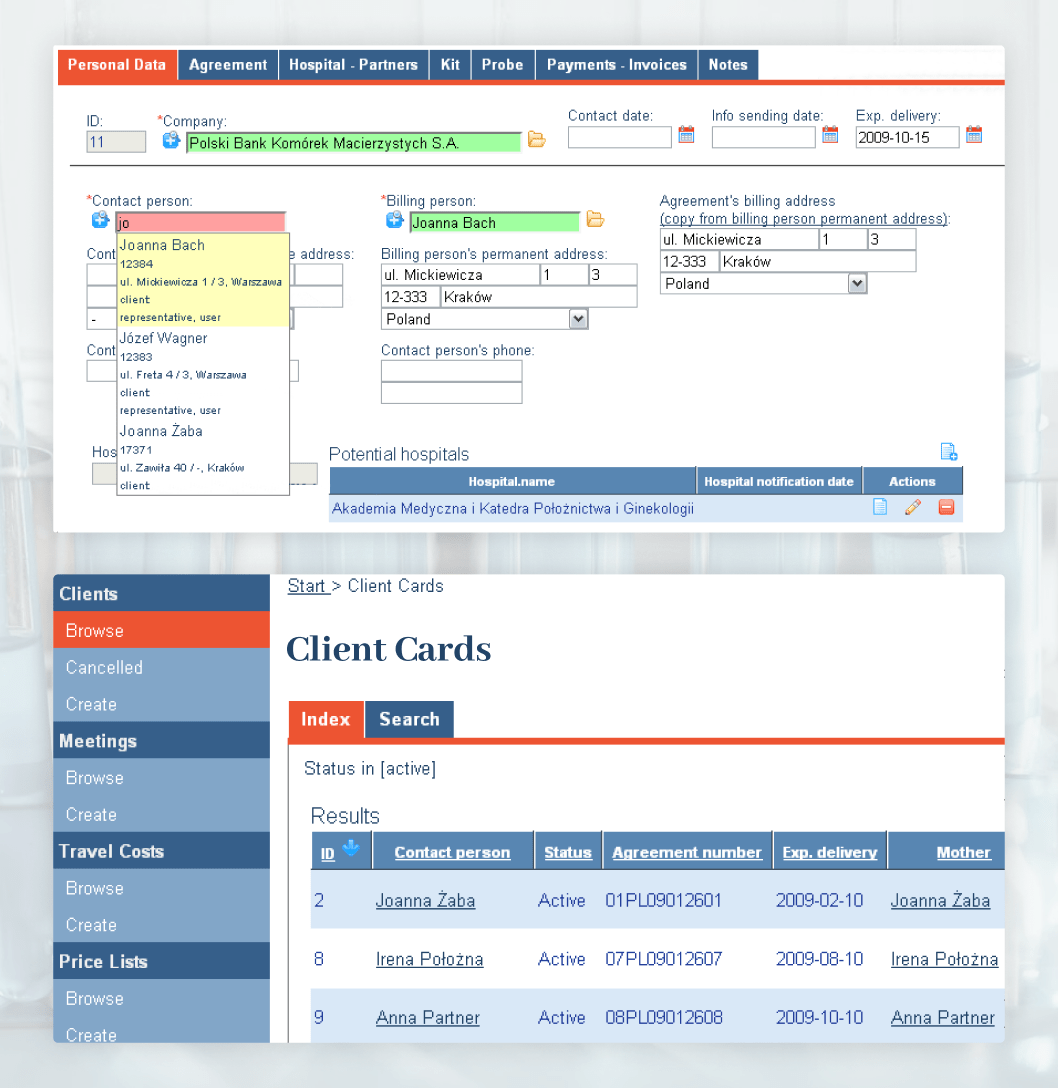
FamiCord needed a flexible and reliable patient database containing personal and medical records to track an increasing number of customers. Before devising an efficient solution with Cledar, the employees had to use generic textual fields for important data, which resulted in an unstructured format and only increased the need for a coherent CRM.
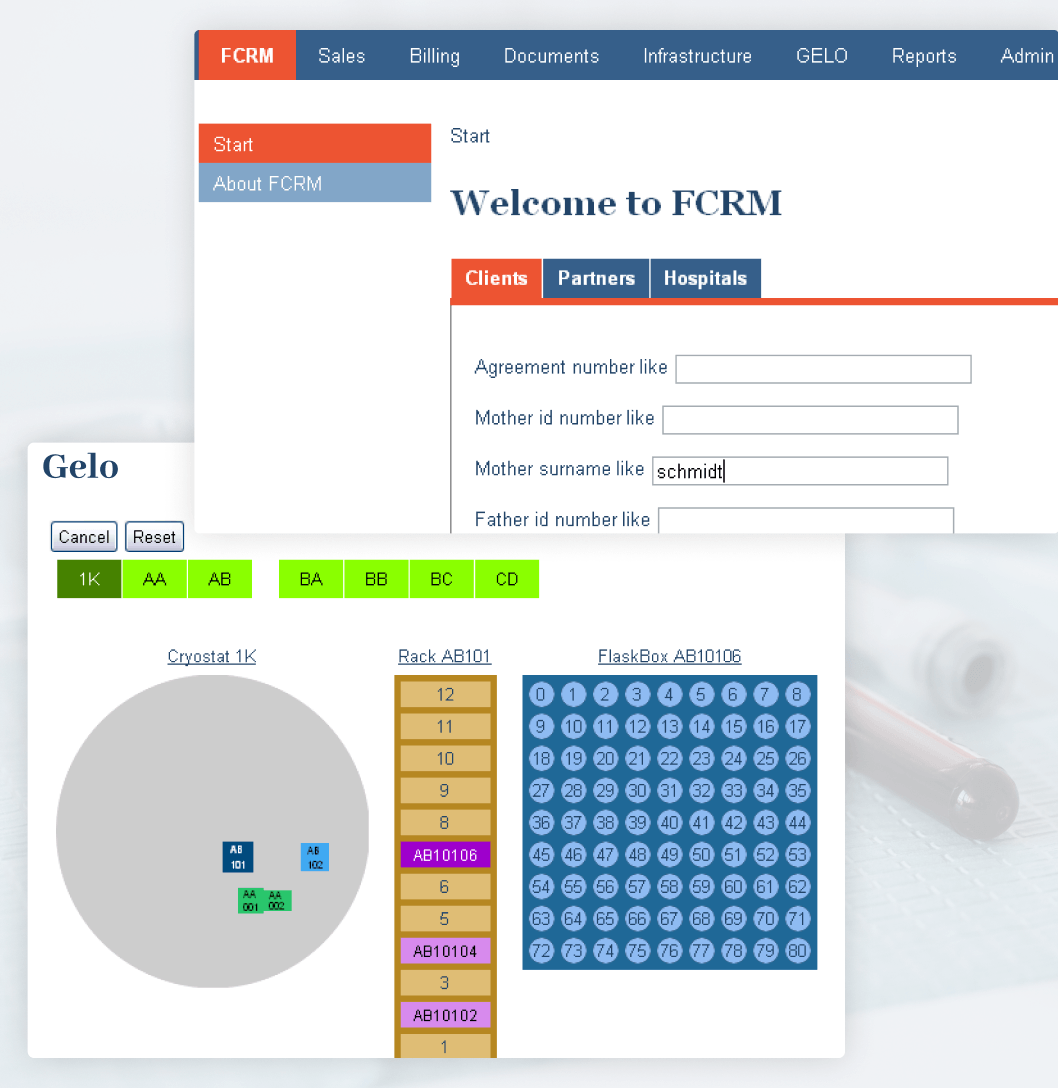
FamiCord labs also required a proper system with automated alarms for failure detection and monitoring the environmental conditions of their cryogenic storage in numerous labs around Europe. This challenge was important to overcome, since it was a threshold requirement to get accreditation from recognized bodies like the Association for the Advancement of Blood & Biotherapies.
Challenge
Solution
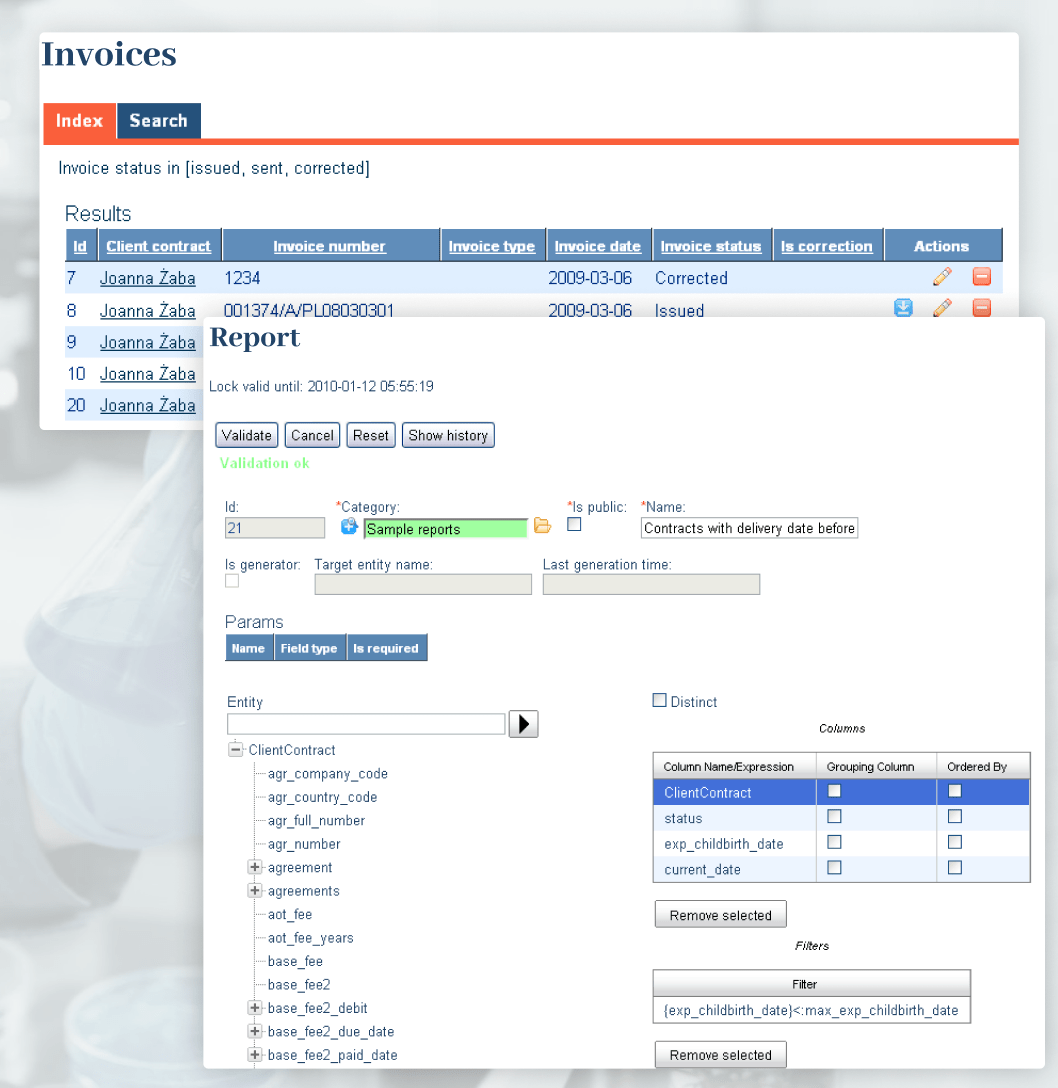
Cledar architected and developed a whole new comprehensive CRM, lab monitoring and maintenance system that fulfilled all the existing and upcoming FamiCord requirements. The patient database was designed to keep track of each patient’s information and provided a complete change tracking and audit trail subsystem, where each change in patient’s data had to be properly annotated and explained before submitting.
The lab management system provided an easy-to-use GUI for previewing cryostats with racks, individual shelves, and it was integrated with environment parameter recorders of various vendors. The data was collected to a central monitoring database and based on threshold alarms generated via the interface to existing alarm systems triggering SMS, audio, and email alarms.
During the deployment stage, Cledar engineered data migration tools to move data from the client’s old solution to the new system.
Solution

Outcome
Cledar started the development of the system for FamiCord when our client was in a rapid dynamic growth phase. They were in the process of acquisition of many of their competitors across Europe with ongoing efforts to consolidate the market. The solution developed by Cledar was shaped by these additional scalability and unification-related requirements and enabled FamiCord to smoothly grow to be a pan-European conglomerate. Our team also supported the client in successfully undergoing the accreditation process of AABB where each component was thoroughly tested: data access control, physical storage safety, and low-latency alarms for environmental condition changes. Currently, FamiCord is the prime cord blood bank in Europe and among the largest in the world.


